Second law of thermodynamics: Second law of thermodynamics states that there is a definite limit to the amount of mechanical energy, which can be obtained from a given quantity of heat energy.
According to Claussius, this lay may be stated as ” It is impossible for a self acting machine working in a cyclic process, to transfer heat from a body at a lower temperature to a body at a higher temperature without the aid of an external agency”.
The second law of thermodynamics has also been stated by Kelvin Planck as ” It is impossible to construct an engine working on a cyclic process, whose sole purpose is to convert heat energy into work.” According to this statement, the second law of thermodynamics is sometimes called as law of degradation of energy.
22) Which of the following statements is correct according to Clausis statement of the second law of thermodynamics ?
Answer is:
It is impossible to transfer heat from a body at a lower temperature to a body at a higher temperature,without the aid of an external source.
Explanation:
KPSC Water Resource Department Assistant Engineer Mechanical Question Paper 2017 with Answer Key
For more MCQ's Click HereRelated KPSC Water Resource Department Assistant Engineer Mechanical Question Paper 2017 with Answer Key
Answer is:
total energy measured with respect to the datum passing through the bottom of the channel
Answer is:
The length of divergent portion in a venturimeter is equal to the convergent portion.
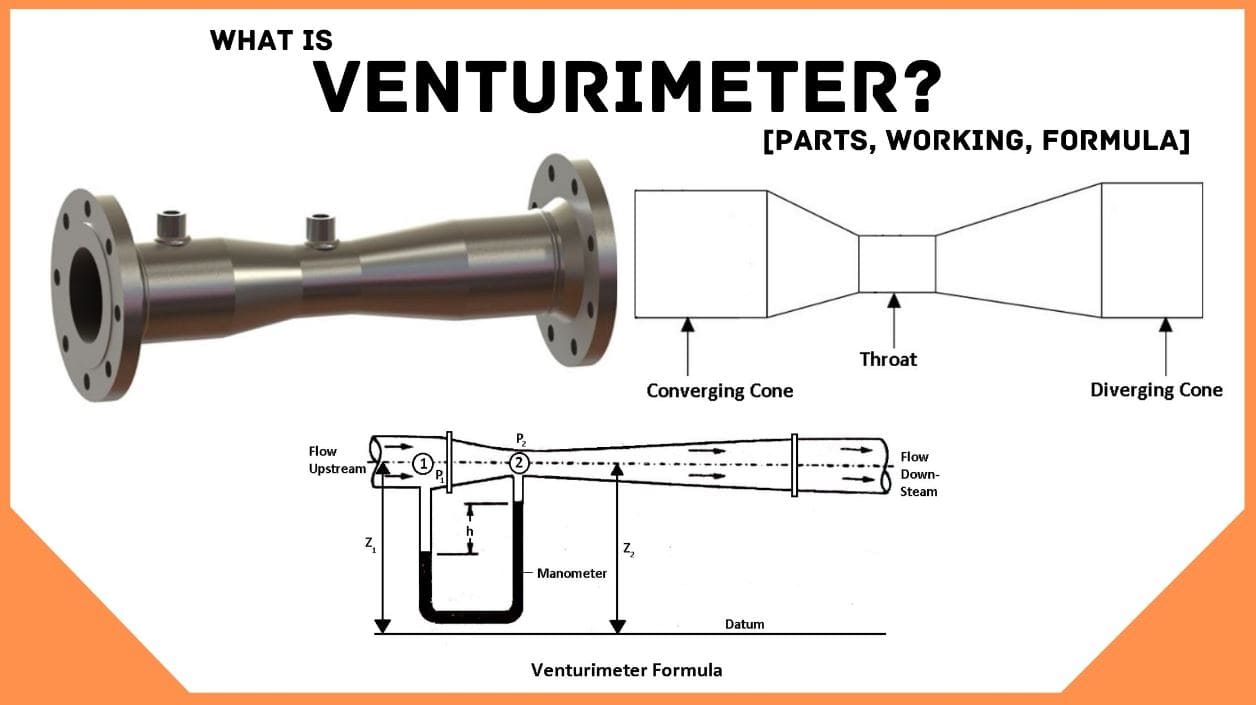
Answer is:
equal volumes of all gases, at the same temperature and pressure, contain equal number of molecules.
Explanation:
Avagadro's Law states, "Equal volumes of all gases, at the same temperature and pressure, contain equal number of molecules."
Thus, according to Avogadro's law, 1 m3 of oxygen (O2) will contain the same number of molecules as 1 m3 of hydrogen (H2) when the temperature and pressure is the same.
Answer is: