Avagadro's Law states, "Equal volumes of all gases, at the same temperature and pressure, contain equal number of molecules."
Thus, according to Avogadro's law, 1 m3 of oxygen (O2) will contain the same number of molecules as 1 m3 of hydrogen (H2) when the temperature and pressure is the same.
25) According to Avogadro's law
Answer is:
equal volumes of all gases, at the same temperature and pressure, contain equal number of molecules.
Explanation:
KPSC Water Resource Department Assistant Engineer Mechanical Question Paper 2017 with Answer Key
For more MCQ's Click HereRelated KPSC Water Resource Department Assistant Engineer Mechanical Question Paper 2017 with Answer Key
Answer is:
L = (2/3)(J + 2)
Answer is:
Bernoulli's equation
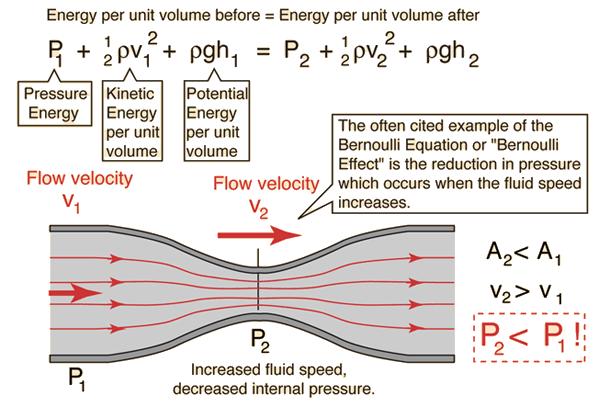
Answer is:
D-Alembert's principle
Answer is: