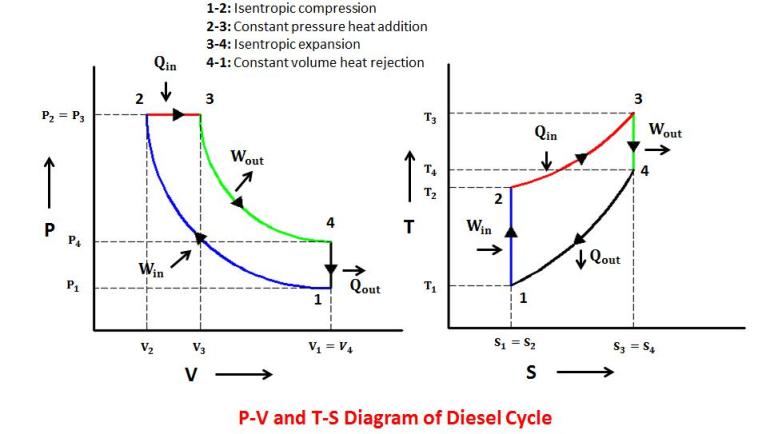
The Diesel cycle is a combustion process of a reciprocating internal combustion engine. In it, fuel is ignited by heat generated during the compression of air in the combustion chamber, into which fuel is then injected, hence Diesel engines are called compression ignition engines.
A Diesel cycle consists of one constant pressure, one constant volume and two isentropic processes.
2) A cycle consisting of one constant pressure, one constant volume and two isentropic processes is known as
Diesel cycle
Related Thermodynamics MCQ with Answers
very low
The efficiency and work ratio of a simple gas turbine cycle are very low.
specific heat at constant volume
The specific heat of a substance my be broadly defined as the amount of heat required to raise the temperature of its unit mass through 1 degree. All the liquids and solids have one specific heat only. But a gas can have any number of specific heats depending upon the conditions, under which it is heated.
Specific heat at constant volume is the amount of heat required to raise the temperature of a unit mass of a gas throught 1 degree, when it is heat at constant volume.
Specific heat at constant pressure is the amount of heat required to raise the temperature of a unit mass of a gas through 1 degree, when it is heated at constant pressure.
The ratio of specific heat at constant pressure Cp and specific heat at constant volume Cv is always more than one.
Right
An irreversible process is a process that cannot return both the system and the surroundings to their original conditions. That is, the system and the surroundings would not return to their original conditions if the process was reversed. There is a loss of heat in an irreversible process.
For example, an automobile engine does not give back the fuel it took to drive up a hill as it coasts back down the hill. There are many factors that make a process irreversible. Four of the most common causes of irreversibility are friction, unrestrained expansion of a fluid, heat transfer through a finite temperature difference, and mixing of two different substances. These factors are present in real,irreversible processes and prevent these processes from being reversible.
all of the above
Adiabatic process or Isentropic process:
A process, in which the work substance neither receives nor gives out heat to its surroundings, during its expansion or compression is called an adiabatic process. This will happen when the working substance remains thermally insulated, so that no heat enters or leaves it during the process. It is thus obvious, that in an adiabatic process no heat leaves or enters the gas, the temperature of the gas changes, as the work is done at the cost of internal energy and the change in internal energy is equal to the work done.
An adiabatic process is one in which
* No heat enters or leaves the gas
* The temperature of the gas changes
* The change in internal energy is equal to the mechanical work done