depends upon the force responsible
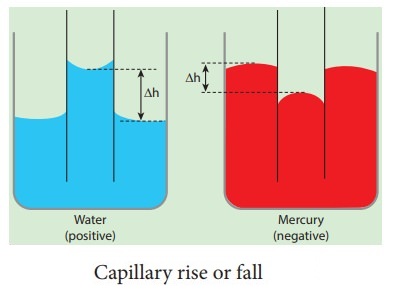
Capillary rise or capillarity is a phenomenon in which liquid spontaneously rises or falls in a narrow space such as a thin tube or in the voids of a porous material. Surface tension is an important factor in the phenomenon of capillarity.
Three main variables that determine whether a liquid possesses capillary action are:
Cohesive force: It is the inter-molecular bonding of a substance where its mutual attractiveness forces them to maintain a certain shape of the liquid.
Surface tension: This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. The surface tension is measured in Newton/meter.
Adhesive force: When forces of attraction between unlike molecules occur, it is called adhesive forces.