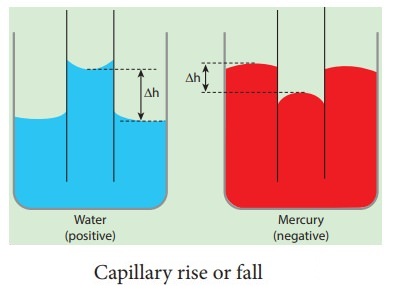
Capillary rise or capillarity is a phenomenon in which liquid spontaneously rises or falls in a narrow space such as a thin tube or in the voids of a porous material. Surface tension is an important factor in the phenomenon of capillarity.
Three main variables that determine whether a liquid possesses capillary action are:
Cohesive force: It is the inter-molecular bonding of a substance where its mutual attractiveness forces them to maintain a certain shape of the liquid.
Surface tension: This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. The surface tension is measured in Newton/meter.
Adhesive force: When forces of attraction between unlike molecules occur, it is called adhesive forces.
83) Capillary rise and fall
Answer is:
Are due to surface tension of the liquid and the tube material
Explanation:
KPSC Water Resource Department Junior Engineer Mechanical Question Paper 2017 with Answer Key
For more MCQ's Click HereRelated KPSC Water Resource Department Junior Engineer Mechanical Question Paper 2017 with Answer Key
Answer is:
Mechanical efficiency
Answer is:
The steam is expanded in nozzles only and there is a pressure drop and heat drop.
Answer is: