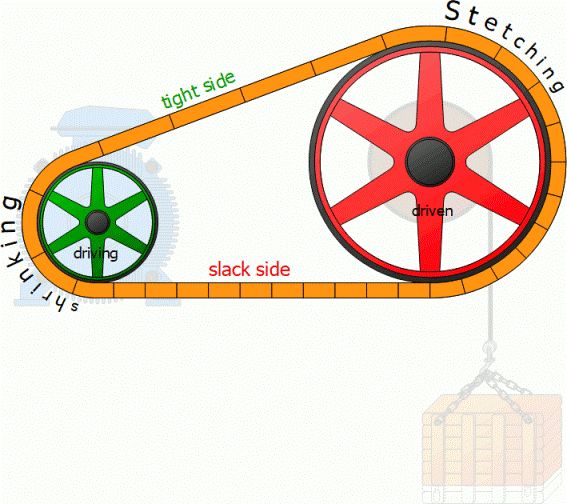
1)Conduction: Conduction is a process of heat transfer from one particle of the body to another in the direction of fall of temperature. The particles themselves remain in fixed position relative to each other. The heat transfer in a metal rod is by conduction.
2)Convection: Convection is a process of heat transfer from one particle of the body to another by convection current. In this case, the particles of the body move relative to each other. The heat transfer, in case of liquids and gases, takes place according to convection.
3)Radiation: Radiation is a process of heat transfer from a hot body to a cold body, in a straight line, without affecting the intervening medium. The heat of sun reaches to us according to radiation.
50) Heat transfer in liquid and gases takes place by
convection
Related KPCL JE Mechanical Question Paper - 2018 with Answer Key
Zero
Different types of thermodynamic processes are
1.Constant Volume process or Isochoric Process
2. Constant Pressure Process or Isobaric Process
3. Hyperbolic process
4. Constant Temperature process or Isothermal process
5. Adiabatic process or isentropic process
6. Polytropic process
7. Free expansion process
8. Throttling process
Constant volume process or isochoric process:
When the gas is heated at a constant volume, its temperature and pressure will increase. Since there is no change in its volume, no external work is done by the gas. All the heat supplied is stored in the body of the gas in the form of internal energy. It may be noted that this process is governed by Gay Lussac law.
Constant pressure process or isobaric process:
When the gas is heated at a constant pressure, its temperature and volume will increase. Since there is a change in its volume, the heat supplied is utilized in incresing the internal inergy of the gas, and also for doing some external work. It may be noted that this process is governed by Charles law.
Hyperbolic process:
A process, in which the gas is heated or expanded in such a way that the product of its pressure and volume remains constant, is called a hyperbolic process.
This process is governed by Boyle’s law.
Constant temperature process or Isothermal process:
A process, in which the temperature of the working substance remains constant during its expansion or compression, is called a constant temperature process or isothermal process. This will happen when the working substance remains in a perfect thermal contact with the surroundings, so that the heat ‘sucked in’ or ‘squeezed out’ is compensated exactly for the mechanical work done by, or on the gas respectively. It is thus obvious that in an isothermal process there is no change in temperature and no change in internal energy.
Adiabatic process or Isentropic process:
A process, in which the work substance neither receives nor gives out heat to its surroundings, during its expansion or compression is called an adiabatic process. This will happen when the working substance remains thermally insulated, so that no heat enters or leaves it during the process. It is thus obvious, that in an adiabatic process no heat leaves or enters the gas, the temperature of the gas changes, as the work is done at the cost of internal energy and the change in internal energy is equal to the work done.
Polytropic process:
The polytropic process is also known as the general law of the expansion and compression of gases.
Free expansion process:
A free expansion occurs when a fluid is allowed to expand suddenly into a vacuum chamber through an orifice of large dimensions. In this process, no heat is supplied or rejected and no external work is done. Hence the total heat of the fluid remains constant. This type of expansion may also be called as constant total heat expansion.
Throttling Process:
when a perfect gas is expanded through an aperture of minute dimensions, such as a narrow throat or a slightly opened valve, the process is termed as throttling process. During this process, no heat supplied or rejected and also no external work is done. Moreover, there is no change in temperature, and so the total heat of the fluid remains constant.
Strain
When a system of forces act on a body, it undergoes some deformation. This deformation per unit length is known as unit strain or simply a strain.
Mathematically
Strain = (Change in Length)/(Original Length)
Primary Strain or Linear Strain The deformation of the bar, per unit length in the direction of the force is known as primary or linear strain.
Secondary Strain or Lateral Strain Every direct stress is always accompanied by a strain in its own direction, and an opposite kind of strain in every direction, at right angles to it. Such strain is known as secondary or lateral strain.
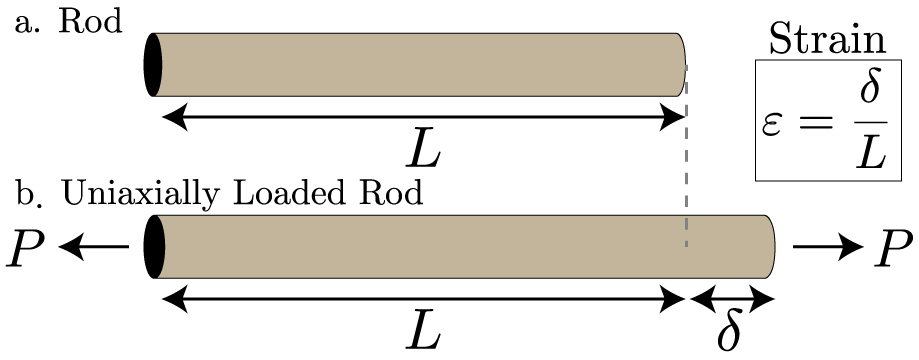
Uneven extensions and contractions due to varying tension
In Belt drives when the belt passes from the slack side to the tight side a certain portion of the belt extends. Similarly when the belt passes from the tight side to slack side it contracts. Hence creep in belt drive is due to uneven extensions and contractions of the belt when it passes from tight side to slack side.
This phenomenon of alternative stretching and contraction of the belt results in relative motion between the belt and the pulley surface. This relative motion is called creep.