Gases can be converted to liquids by compressing the gas at a suitable temperature.
Gases become more difficult to liquefy as the temperature increases because the kinetic energies of the particles that make up the gas also increase.
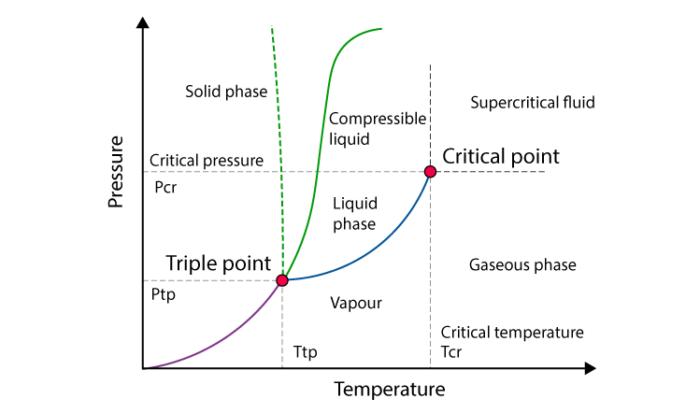
The critical temperature of a substance is the temperature at and above which vapor of the substance cannot be liquefied, no matter how much pressure is applied.
10) Critical temperature is the temperature above which
Answer is:
a gas will never liquefy
Explanation:
Related ISRO Technician-B MRAC Question Paper - 2016 (Set -1) with Answer Key
Answer is:
Copper
Answer is: