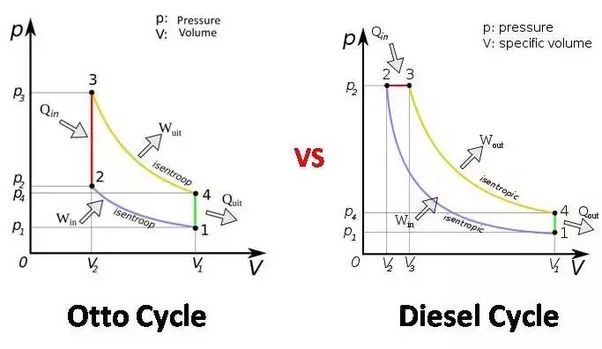
Diesel cycle is called constant pressure cycle because the heat addition process in diesel cycle is done at constant pressure whereas petrol cycle is called constant volume cycle (Otto Cycle) as here the heat addition is done at constant volume. (Heat rejection in both the cycles is done at constant volume).
Constant volume cycle involves Two constant volume and two reversible adiabatic process.
80) Constant volume cycle involves
Two constant volume and two reversible adiabatic process
Related Thermal Engineering MCQ with Answers
Conversion of heat into work
Different thermodynamics laws are
1. Zeroth law of thermodynamics
2. First law of thermodynamics
3. Second law of thermodynamics
Zeroth law of thermodynamics : Zeroth law of thermodynamics states that, when two bodies are in thermal equilibrium with a third body. They are also in thermal equilibrium with each other.
First law of thermodynamics: First law of thermodynamics states that, Heat and mechanical work are mutually convertible. According to this law, a definite amount of mechanical work is needed to produce a definite amount of heat and vice versa.
First law of thermodynamics also states that energy can neither be created nor destroyed, through it can be transformed from one form to another. According to this law, the energy due to heat supplied must be balanced by external workdone plus the gain in internal energy due to rise in temperature.
Second law of thermodynamics: Second law of thermodynamics states that there is a definite limit to the amount of mechanical energy, which can be obtained from a given quantity of heat energy.
According to Claussius, this lay may be stated as ” It is impossible for a self acting machine working in a cyclic process, to transfer heat from a body at a lower temperature to a body at a higher temperature without the aid of an external agency”.
The second law of thermodynamics has also been stated by Kelvin Planck as ” It is impossible to construct an engine working on a cyclic process, whose sole purpose is to convert heat energy into work.” According to this statement, the second law of thermodynamics is sometimes called as law of degradation of energy.