Classification of thermodynamic systems are
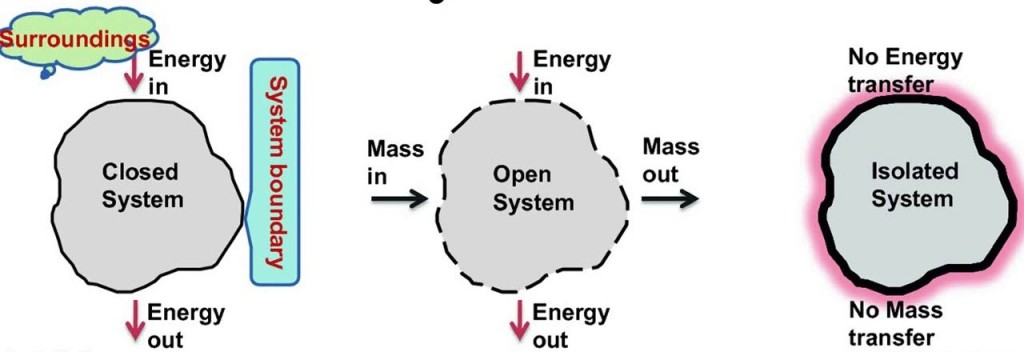
1. Closed system: Closed system is one in which boundary of the system does not allow the matter (mass) to cross it, then it is known as closed system. Here the system contains fixed or constant amount of matter (mass). Energy can cross the boundary. Heat and work are the only ways in which energy can be transferred between a closed system and its surroundings.
2. Open system: Open system is one in which boundary of the system allows the matter (mass) to flow into or out of the system, then it is known as open system. In open system matter (Mass) crosses the boundary of the system. Heat and work may also cross the boundary.
3. Isolated system: Isolated system is one in which boundary of the system does not allow the matter (mass) or the energy to flow into or out of the system, then it is known as isolated system. An isolated system in one which is completely uninfluenced by the surroundings. It is of fixed mass and no heat or work crosses the boundary of the system.
2) An open system is one in which
Both the heat and work as well as mass of the working substance cross the boundary of the system.
Related Thermal Engineering MCQ with Answers
Volume
Extensive properties The properties of the system, whose value for the entire system is equal to the sum of their values for the individual parts of the system, are called extensive properties.
For example, total volume, total mass and total energy of a system are extensive properties.
Intensive properties The properties of the system, whose value for the entire system is not equal to the sum of their values for the individual parts of the system, are called intensive properties.
For example, temperature, pressure and density of a system are intensive properties.
Joule
Zeroth law of thermodynamics
Zeroth law of thermodynamics : Zeroth law of thermodynamics states that, when two bodies are in thermal equilibrium with a third body. They are also in thermal equilibrium with each other.