Adiabatic process or Isentropic process:
A process, in which the work substance neither receives nor gives out heat to its surroundings, during its expansion or compression is called an adiabatic process. This will happen when the working substance remains thermally insulated, so that no heat enters or leaves it during the process. It is thus obvious, that in an adiabatic process no heat leaves or enters the gas, the temperature of the gas changes, as the work is done at the cost of internal energy and the change in internal energy is equal to the work done.
An adiabatic process is one in which
* No heat enters or leaves the gas
* The temperature of the gas changes
* The change in internal energy is equal to the mechanical work done
19) An adiabatic process is one in which
All of the above
Related Thermal Engineering MCQ with Answers
Two isothermal and two isentropic processes
The Carnot cycle is a theoretical thermodynamic cycle that consists of four reversible processes, which are:
Isothermal Expansion: The working substance (e.g. a gas) is placed in contact with a heat reservoir at a high temperature, and heat is transferred to the working substance, causing it to expand while maintaining a constant temperature. This is a reversible process because the heat is transferred slowly enough to maintain thermal equilibrium throughout the expansion.
Adiabatic Expansion: The working substance is now thermally isolated (i.e., no heat can enter or leave the system), but it continues to expand, causing its temperature to decrease. This process is reversible because no heat is exchanged, and the expansion is slow enough to maintain thermal equilibrium.
Isothermal Compression: The working substance is now placed in contact with a heat reservoir at a low temperature, and heat is transferred out of the system, causing it to compress while maintaining a constant temperature. This is a reversible process because the heat is transferred slowly enough to maintain thermal equilibrium throughout the compression.
Adiabatic Compression: The working substance is now thermally isolated, but it continues to be compressed, causing its temperature to increase. This process is reversible because no heat is exchanged, and the compression is slow enough to maintain thermal equilibrium.
The Carnot cycle is a highly efficient cycle that represents the maximum efficiency that any heat engine operating between two temperatures can achieve, given that it operates in a reversible manner.

All the heat engines are based on Carnot cycle.
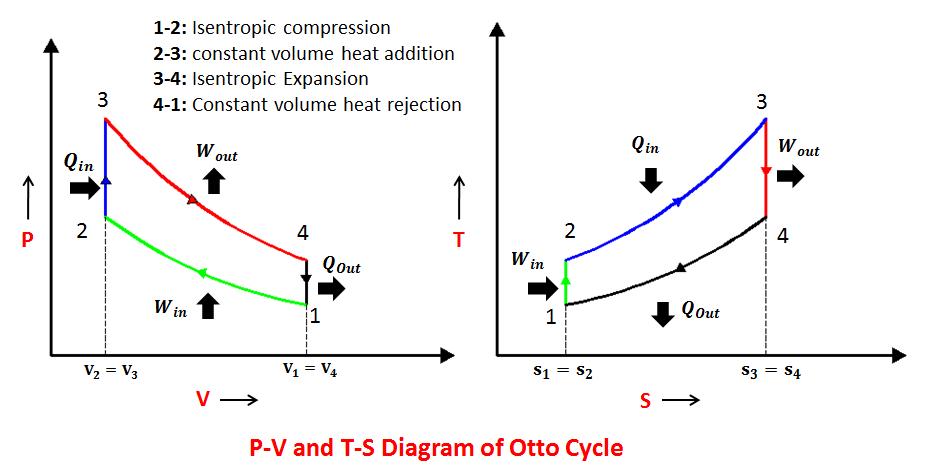
Two constant volume and two isentropic processes
Otto cycle consists of two isentropics and two constant volume processes.