First law of thermodynamics: First law of thermodynamics states that, Heat and mechanical work are mutually convertible. According to this law, a definite amount of mechanical work is needed to produce a definite amount of heat and vice versa. According to First law of thermodynamics Total energy of a system remains constant.
First law of thermodynamics also states that energy can neither be created nor destroyed, through it can be transformed from one form to another. According to this law, the energy due to heat supplied must be balanced by external workdone plus the gain in internal energy due to rise in temperature.
31) In the first law of thermodynamics, the total energy of the system remains constant.
Answer is:
Right
Explanation:
Related Thermodynamics MCQ with Answers
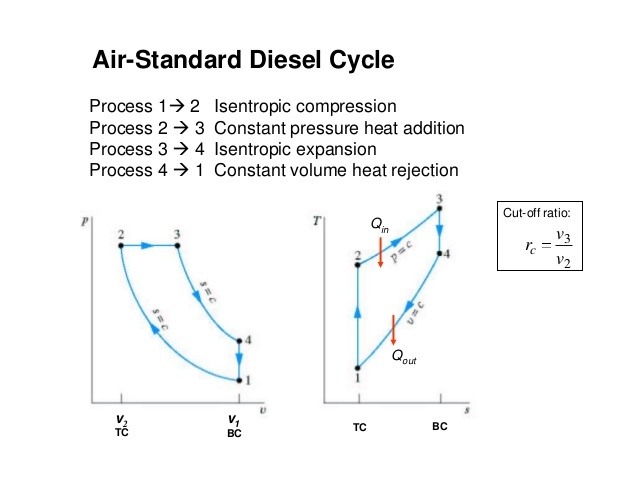
Answer is:
decrease in cut-off
Explanation:
The efficiency of Diesel cycle increases with decrease in cut-off.
Cutoff ratio is the ratio of the cylinder volumes after and before the combustion process. As the cutoff ratio decreases, the efficiency of the diesel cycle increases.
Answer is:
directly proportional to
Answer is: