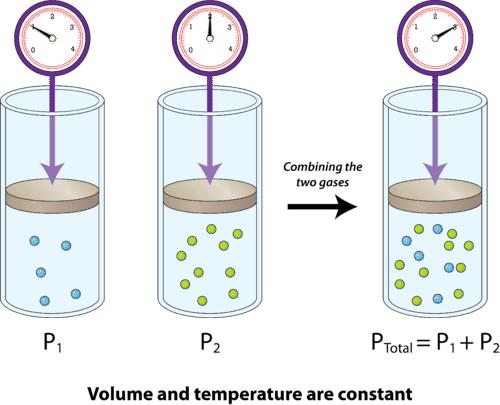
According to Dalton's law of partial pressures, the total pressure by a mixture of gases is equal to the sum of the partial pressures of each of the constituent gases. The partial pressure is defined as the pressure each gas would exert if it alone occupied the volume of the mixture at the same temperature.
10) According to Dalton's law of partial pressures, (where pb = Barometric pressure, pa = Partial pressure of dry air, and pv = Partial pressure of water vapour)
Answer is:
Pb = pa + pv
Explanation:
Related Heat Transfer Refrigeration and Air Conditioning MCQ with Answers
Answer is:
the ratio of actual mass of water vapour in a given volume of moist air to the mass of water vapour in the same volume of saturated air at the same temperature and pressure
Answer is:
Incorrect
Answer is:
ammonia and water
Answer is: